How Does Lime Affect a Pond?
You may have heard of lime being added to gardens or agricultural fields to improve fertility, but lime can also be extremely useful for ponds. Lime is used agriculturally to adjust pH levels in the soil and improve nutrient availability, and it has the same effect when used in ponds. As we’ll discuss below, adding lime serves two primary functions in a pond or a lake.
Adding lime will improve nutrient availability in ponds and buffer the pH against drastic swings between day and night hours. Improving nutrient availability has several benefits including boosting the base of the pond food chain and providing a natural control for subsurface weeds. Reducing wide swings in the pH of the pond water will ensure that fish are not stressed and they continue to grow as they should.
From a chemistry perspective, lime increases the alkalinity and hardness of the pond water. Alkalinity refers to the concentration of bases in the water. These include bicarbonates, carbonates, and hydroxides. Hardness refers to the concentration of salts in the water, mainly calcium and magnesium. When we perform an electrofishing survey, we always collect a water sample and test the alkalinity and hardness of the pond water. The results of the water quality test often help to explain any deficiencies we might find in the fish populations.
Improving Nutrient Availability
The soils at the bottom of ponds are generally acidic, especially in ponds with a hard clay bottom. These acidic soils contain nutrients that are bound to the soil, meaning they are not easily leached into the pond water. As long as the pond water remains relatively acidic, those nutrients will remain in the soil.
Phosphorous is one of the primary nutrients that can be bound in pond sediments, and phosphorous is an essential major nutrient for phytoplankton. These are microscopic photosynthetic organisms that live in the pond water and form the bottom of the pond food chain. The basic pond food chain starts with phytoplankton which are then consumed by zooplankton. The zooplankton are then consumed by sunfish (bluegill, shellcracker) which are then consumed by predators like largemouth bass.
Phosphorous is generally considered the most limited nutrient in ponds. This is because most of it remains bound in the sediment of acidic ponds. As a result, phosphorous availability is greatly reduced when the pH of the pond water is lower. Adding lime will increase the pH of the pond water and help to release the bound phosphorous into the pond water. This increases the nutrient concentration in the water and helps to feed those essential phytoplankton at the bottom of the food chain.
Releasing bound nutrients through liming not only stimulates the pond food chain, but it also can help with subsurface weed control. As phosphorous is released into the water after liming, the phytoplankton populations will boom. This results in a “bloom” which gives a greenish tint to the water surface.
This green bloom on the pond surface prevents lights from reaching the pond bottom. Subsurface weeds thrive in ponds with clear water because light penetrates deeply and feeds those aquatic weeds. By reducing the light penetration into the water with a thriving phytoplankton population, subsurface weeds are much easier to manage.
Buffering pH
All ponds include photosynthetic organisms in the form of phytoplankton and aquatic vegetation. During the day, light triggers photosynthesis to occur in these organisms. This process involves the consumption of carbon dioxide (CO2) and the release of oxygen into the pond water. As such, carbon dioxide concentrations in the pond water are lower during the day when light is present.
In the nighttime hours, plants will not photosynthesize because there’s no light to trigger those chemical reactions in the aquatic organisms. Instead, they will respire. The process of respiration in plants consumes oxygen and releases carbon dioxide into the water. As such, carbon dioxide concentrations are higher during the evening hours when plants are not actively photosynthesizing.
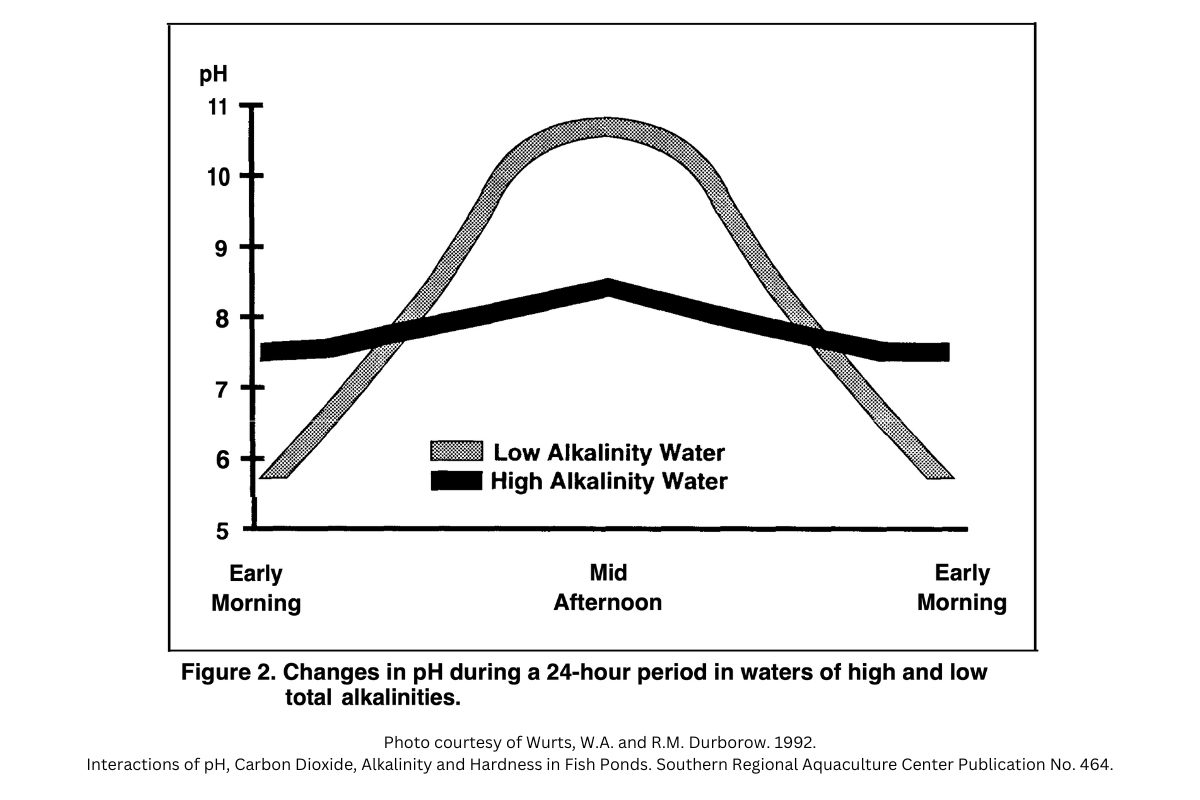
Carbon dioxide acts as an acid in water and decreases the pH of the water. As a result, this cycle of photosynthesis and respiration causes pond water pH to fluctuate between daytime and nighttime hours. During the day when photosynthesis is the dominant process, water pH will be higher. During the night when respiration is the dominant process, water pH will be lower.
These daily swings in pH can be quite stressful on the fish populations in cases where pH goes from very acidic to very alkaline in a 24 hour span. It can even result in massive fish kills in some cases. Adding lime to a pond increases the alkalinity of the water and reduces the severity of these daily pH swings. As a result, aquatic organisms are not stressed and their growth will not be compromised.


